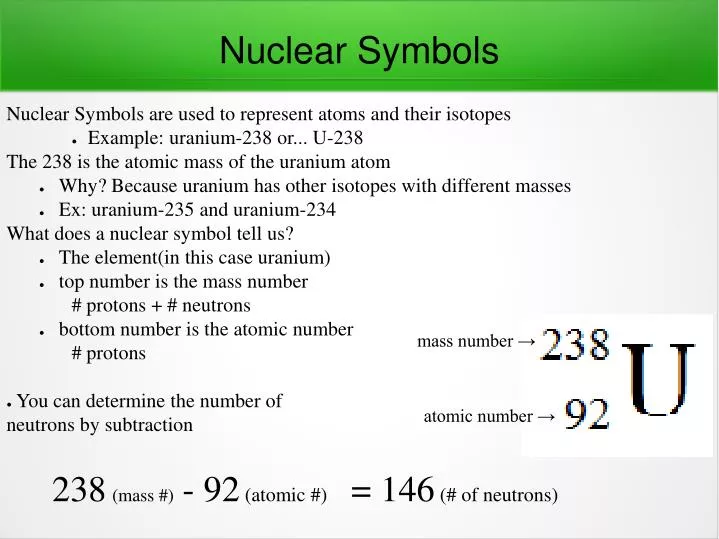
- Uranium Atomic Number And Mass Number
- Mass Number For Uranium 235
- Uranium-239 Mass Number
- Uranium Mass Number And Atomic Number
Further data for naturally occuring isotopes of uranium are listed above. This table gives information about some radiosotopes of uranium, their masses, their half-lives, their modes of decay, their nuclear spins, and their nuclear magnetic moments. Isotope Mass / Da Half-life Mode of decay Nuclear spin Nuclear magnetic moment; 230 U: 230.03393. By surrounding the fissionable material with a suitable neutron 'reflector', the loss of neutrons can reduced and the critical mass can be reduced. By using a neutron reflector, only about 11 pounds (5 kilograms) of nearly pure or weapon's grade plutonium 239 or about 33 pounds (15 kilograms) uranium 235 is needed to achieve critical mass. Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table.A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons.

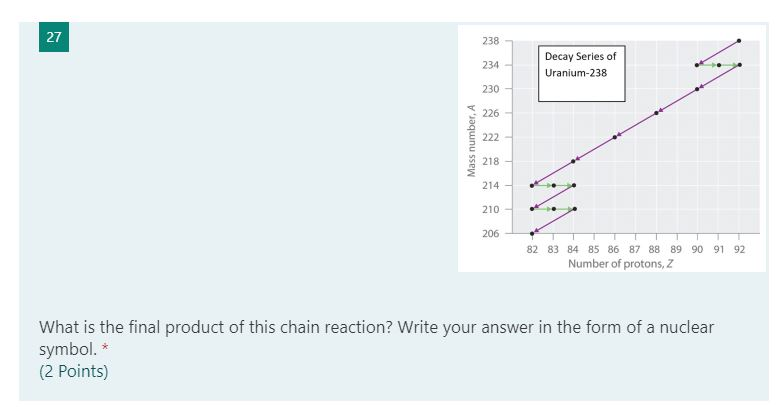
What is the nuclear equation for uranium-238 after alpha radiation is emitted?
1 Answer
Explanation:
Uranium Atomic Number And Mass Number
Uranium-238 produces thorium-234 by alpha decay.
An α-particle is a helium nucleus. It contains 2 protons and 2 neutrons, for a mass number of 4.

During α-decay, an atomic nucleus emits an alpha particle. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less.
Mass Number For Uranium 235

Thus, uranium-238 decays through α-particle emission to form thorium-234 according to the equation:
Uranium-239 Mass Number
Note that the sum of the subscripts (atomic numbers or charges) is the same on each side of the equation.
Also, the sum of the superscripts (masses) is the same on each side of the equation.
Uranium Mass Number And Atomic Number
Related questions